Background
The upper age limit for patients undergoing allogeneic hematopoietic cell transplantation (HCT) has increased over the past several years due to continued improvements in supportive care and increased expertise in management of older recipients of allogeneic HCT. This change is particularly important for hematologic malignancies such as acute myeloid leukemia (AML) where the median age at diagnosis is in the late 60s and HCT provides, in many instances, the only potential chance of cure. While some previous analyses have suggested favorable post-HCT outcomes for older AML patients in general, information is limited about allogeneic HCT for patients aged 70 years or older. Here, we set out to retrospectively analyze post-HCT outcomes for those aged 70+ and compare these outcomes to those aged 60-69 in a large single-center observational study.
Methods
Eligible patients were identified using our Fred Hutchinson Cancer Center database of transplant outcomes. All patients signed consents at time of HCT. Severity of disease was categorized as previously described (McDonald GB et al Ann Intern Med 2020). Kaplan-Meier estimates were used to evaluate overall survival (OS) and relapse-free survival (RFS); cumulative incidence estimates were used to summarize relapse and non-relapse mortality (NRM). Multivariable Cox regression models were fit to compare outcomes across age groups after adjusting for potential imbalances in various factors between groups. In addition, we used a multivariable model for overall mortality (OM) to identify risk factors for death in this population.
Results
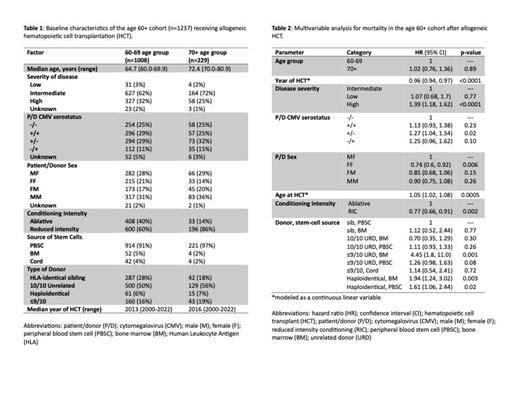
We identified 1237 patients given allogeneic HCT between 2000-2022 aged at least 60 years old, 229 (18.5%) of whom were of age 70+. Baseline characteristics included severity of disease, donor type, patient CMV serostatus, conditioning intensity (Table 1).
The median OS among patients 60-69 was 2.8 years (95% confidence interval, 2.4-3.6 years) and among those 70+, 1.8 years (1.3-2.9 years). Point estimates of OS at 3, 5, and 7 years were 49% (46-52%), 46% (43-49%), and 37% (34-40%), respectively for patients aged 60-69, and 43% (36-49%), 38% (31-45%), and 28% (21-36%), respectively, for patients age 70+. In the subgroup of patients age 75+ (n=29), the 3- and 5-year point estimates of OS were each 41% (22-59%).
The unadjusted hazard ratio (HR, 70+ vs. 60-69) for death was 1.24 (1.04-1.49, p=0.02). However, after adjusting for various factors (Table 2), the adjusted HR for death was 1.02 (0.76-1.36, p=0.90), suggesting the apparent reduction in OS for the 70+ group relative to the 60-69 group was largely due to a higher proportion of adverse risk factors in the 70+ group. Median RFS for the two groups were 1.9 years (1.4-2.3 years) and 1.3 years (0.9-1.8 years), respectively. Similar to the HR for death, the unadjusted HR for RFS failure was significant at 1.23 (1.03-1.46, p=0.02), but the adjusted HR for was no longer significant at 0.96 (0.72-1.26, p=0.74). The unadjusted and adjusted HRs for relapse were 1.10 (0.84-1.44, p=0.51) and 0.77 (0.50-1.17, p=0.22), respectively, and for NRM 1.33 (1.06-1.67, p=.02) and 1.12 (0.77-1.61, p=0.56), respectively. Factors that were demonstrably associated with increased mortality included severity of disease, patient/donor CMV serostatus, conditioning intensity, and type of donor (Table 2).
Conclusions
We report here on one of the largest groups of patients aged 70+ receiving allogeneic HCT in a single center. Our results suggest that allogeneic HCT is feasible and potentially safe in this age group as compared to the group aged 60-69. These results should be encouraging for consideration of patients 70+ for allogeneic HCT. We are in process of complementing our analyses with information on comorbidities.
On the other hand, 7-year OS for all older pts (60-80.9 years old) ranged from 28-37%, highlighting the need for dedicated HCT trials targeting this group of older patients to further improve outcomes.
Disclosures
Percival:Ascentage: Research Funding; Astex: Research Funding; Abbvie: Research Funding; Biosight: Research Funding; BMS: Research Funding; Glycomimetics: Research Funding; Pfizer: Research Funding; Telios: Research Funding. Sorror:JAZZ pharmaceuticals: Consultancy; BlueNote and Massachusetts General Hospital: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal